
On April 22, 2025, after the public announcement of the National Medical Products Administration, the Silk Fibroin Dressing (Registration Certificate Number: 20252140672) developed by Soho Biotech was approved to be listed by the NMPA, and the product was approved for the national registration certificate of Class II medical device. Silk Fibroin Dressing Silk fibroin Dressing, which is made of non-woven fabric impregnated with silk fibroin solution, is suitable for the coverage and care of non-chronic wounds. The launch of this product will bring more choices in the field of wound treatment, and its excellent clinical therapeutic effect will greatly improve the quality of life of patients. Technological Advantages: 1. The 18 amino acids in the composition of silk fibroin are similar to the composition of human skin, and the internal structure of silk is 87% similar to human skin, which makes the film less irritating to human skin, with high biosafety and good biocompatibility. 2. Relative to the certified product silk fibroin wound dressing products, the use of this product can reduce the doctor's operating procedures, reduce the patient's cost of expenditure, and use more convenient, faster, to meet the doctor's needs for a small range of wound dressings. 3. This product is sterile, no preservatives, no flavors, no fluorescents, no coloring, and no chemical additives. About Soho Biotech We has undertaken the special project of National 13th Five-Year Key R&D Plan for Biomaterials Research and Development and Organ Repair Replacement, and has achieved the transformation of scientific and technological achievements in Engineering Preparation Technology and Product Development of Low Immunogenicity Collagen and Silk Fibroin. It holds core patents for silk fibroin (SFM), and has also developed various research and development technologies of new generation mainstream medical materials such as hyaluronic acid, PLA, PLLA, glucan, PCL, peptides, polyethylene, PVA, polyurethane, polytetrafluoroethylene, microcrystalline calcium, chitosan, hydroxyapatite, humanized collagen, etc. We provides technical upgrades and new project reserves services for manufacturers and distributors, with strategic partnerships established globally with listed companies such as Fosun Pharma, Double Crane Pharma, and So-Young Group.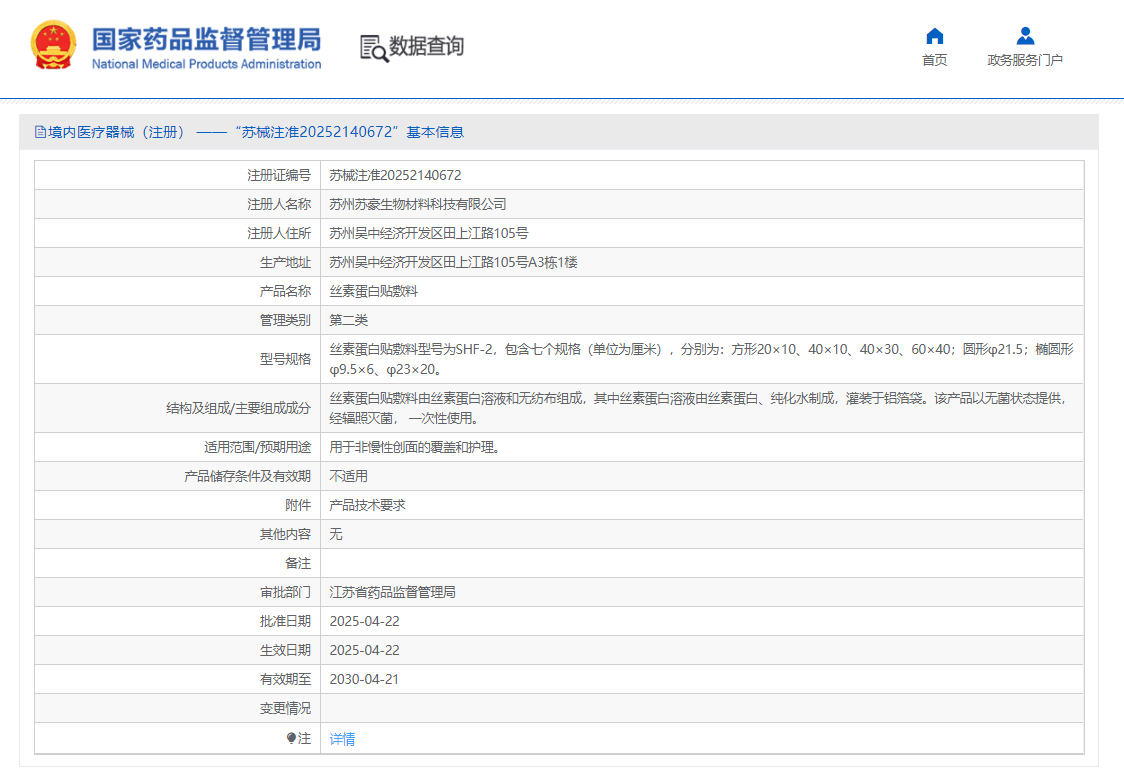

Copyright ©2020 www.suhaobio.com.cn, All Rights Reserved
Jiangsu lCP No. 19075400-1 ![]() Jiangsu Computer Information Network and internet unit registration No. 32050602011157 Qualification Certificate for Medicine Trading Services on the Internet:(Jiangsu)-Non-Operating-2020-0041
Jiangsu Computer Information Network and internet unit registration No. 32050602011157 Qualification Certificate for Medicine Trading Services on the Internet:(Jiangsu)-Non-Operating-2020-0041